
product catalog
The DMEqual implant features an internal hexagonal conical connection design that offers a more versatile approach to abutment restoration, catering to various clinical needs. Its internal conical connection effectively reduces the risk of inflammation, ensuring better long-term stability. The conical apex design allows for secure fixation in double cortical bone, while the four-blade self-tapping groove demonstrates excellent self-tapping performance in cases of insufficient preparation. Coupled with SLA surface treatment and groove design, this enhances the implant’s initial stability and biocompatibility, making it an ideal choice for your needs.
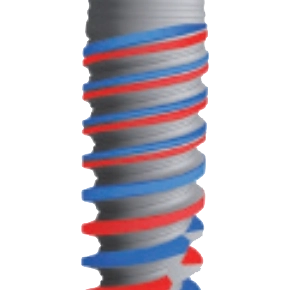
Faster implantation and optimized surgical experience

Abutment prosthetics are more diversified with hexagonal interlocking to reduce inflammation effectively
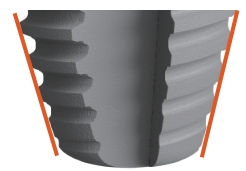
Double cortical bones fixation is permitted with the tapered root tip Four-edged tapping grooves have good tapping effect when the width or the length of the hole are insufficient


Tight protective layer is formed with soft tissue attachment
Flexible for a wide range of bones
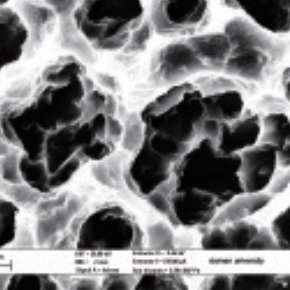
Increase the contact area and stability

Effectively reduces bone resorption around the implant and forms a stable soft tissue closure
As a professional dental implant manufacturer, we produce implants from high-quality titanium using advanced manufacturing techniques. Fully compatible with NobelParallel CC, our implants come with a lifetime warranty for long-term reliability and peace of mind.

The DMEqual implant features a 12° tapered connection that effectively disperses stress and provides a tight seal to prevent bacterial growth. Its excellent load distribution and sealing properties ensure stability and safety during use. The internal hexagonal connection enhances anti-rotation capability, while the hexagonal lock design allows for versatile restoration directions. Additionally, the tapered connection reduces inflammation risk, offering patients a more comfortable experience and improved treatment outcomes.
The DMEqual implant features a surface treatment that uses mixed particle size sandblasting to create a unique, uniformly structured micro-level morphology with deep pores, enhancing the contact area with bone tissue. This multi-level micro surface undergoes high-temperature activation, resulting in a thin, crystalline titanium dioxide layer that significantly reduces the contact angle and promotes biomineralization post-implantation. This treatment facilitates both short-term loading and long-term osseointegration, ensuring a successful and stable implant experience.


Our implants are made from high-quality Cold Working Titanium Grade 4. This material is produced by cold-working Grade 4 pure titanium, a process that not only retains the biocompatibility of pure titanium but also significantly enhances its mechanical strength. With exceptional biocompatibility, it integrates well with surrounding tissues in the body without causing adverse reactions. Additionally, its high strength allows the implant to withstand greater bite forces, making it particularly suitable for dental restorations that need to endure heavy chewing pressure.
Under the electron microscopy 150x and 20000x, the gap between DentalMaster and abutment is smaller than 0.2micron,lower than similar products in the industry, shows the processing accuracy is higher, therefore anti-fatigue is better, anti-deformation is better, duration period is longer

We are a large-scale medical manufacturing facility covering over 100,000 square meters, equipped with advanced production lines and strict quality control systems. Our company is ISO 13485 certified, ensuring compliance with international medical standards. The DMEqual implant is CE certified for sale in Europe and also holds registration certificates for markets such as Indonesia and Malaysia, making it suitable for global clinical applications.

CE certification (click to view)

ISO13485 certification (click to view)

DENTALMASTER SLA Dental Implant System_Product Approval_Indonesia Exp. 12102025 (click to view)

Product license 67-2-2-2-0004127_DentalMaster Dental Implant System (click to view)
We invite distributors, hospitals, and dental clinics to join us in creating more smiles, fueled by a shared passion for dental implants.
DentalMaster is a company specializing in implantation dentistry. Brands such as Nobel Biocare, Short Bicon, Straumann, Zimmer, Megagen, Dentium, and Hiossen are registered trademarks of their respective companies. DentalMaster has no commercial affiliation with these companies. Mentioning these brands is solely for the purpose of accurately identifying compatible implants, instruments, abutments, and components offered by DentalMaster.
Quick Links
Products
Contact Us
Copyright © 2024 DentalMaster Dental Instrument Suppliers CE/ISO13485. All Rights Reserved.