Guided Bone Regeneration (GBR) technology is an efficient and convenient method for bone augmentation, widely used in alveolar ridge reconstruction for implant-related bone defects. With its excellent mechanical properties and biocompatibility, the introduction of titanium mesh has greatly expanded the scope of GBR applications, enabling the technique to successfully address larger bone defects and achieve significant bone augmentation results in a stable manner.
Currently, GBR technology combined with titanium mesh covers a wide range of clinical applications and involves various delicate clinical procedures. During this process, practitioners can flexibly choose appropriate bone graft materials, titanium mesh coverage methods, and fixation strategies based on the specific needs of each case to meet the personalized treatment requirements of different patients. With advances in technology, titanium mesh-based GBR has also seen significant progress in digital technology and material modifications. This article aims to comprehensively explore the unique properties of titanium mesh, compare it with other barrier membranes, and delve into its clinical applications in bone augmentation, including its successful use in various complex cases. Additionally, common complications associated with the use of titanium mesh will be discussed.
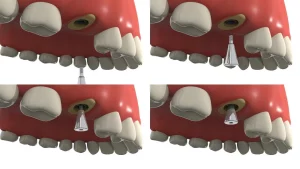
Guided Bone Regeneration (GBR) Technology and Titanium Mesh
Guided Bone Regeneration (GBR) technology has become one of the mainstream choices for repairing alveolar bone defects, thanks to its simplicity, relatively low technical barrier, excellent bone stability, and remarkable multi-directional osteogenic potential.
The core concept of GBR technology lies in leveraging the differences in cell migration rates. Through a carefully designed barrier membrane, GBR effectively prevents the invasion of epithelial and connective tissue cells into the bone defect area, thereby providing a clear and unobstructed pathway for osteoblasts to prioritize bone induction and regeneration within the defect area. At the same time, the carefully selected bone graft materials act as a scaffold, firmly anchoring in the bone defect area and guiding osteoblasts and bone cells to collaborate in creating new bone.
It is important to note that in the clinical practice of GBR technology, the space support function in the bone defect area may be more critical than selective cell isolation. If there is insufficient support in the grafting area, the bone defect may shift due to local stress, leading to collapse in the bone augmentation area and compromising the treatment results. Therefore, the barrier membrane in GBR technology must not only have good biocompatibility but also provide sufficient stiffness, support, and retention.
However, traditional barrier membranes (such as absorbable collagen membranes or non-absorbable expanded polytetrafluoroethylene (ePTFE) membranes) while effective at cell selective isolation, are relatively soft and cannot provide sufficient retention and protection for the bone regeneration area. When faced with large bone defects, traditional membranes lack the rigidity to maintain a stable and suitable bone regeneration space, and may even affect blood supply due to micro-movement.
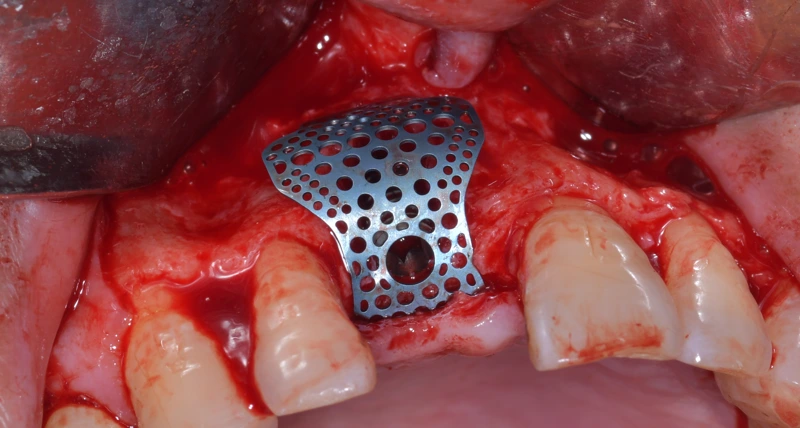
In this context, titanium mesh, with its excellent mechanical properties and osteoconductive performance, has shone in clinical applications involving severe vertical or horizontal bone defects in the alveolar bone. Numerous studies show that titanium mesh not only provides strong support and protection for the bone regeneration area but also effectively promotes the formation and regeneration of new bone.
As an essential technology in the field of oral implantology, the clinical value of GBR technology is unquestionable. The introduction of titanium mesh undoubtedly injects new vitality and possibilities into GBR, opening new pathways for the repair of alveolar bone defects.
Titanium Mesh: Performance and Characteristics
Titanium mesh excels in mechanical performance, biological properties, and osteoconductive characteristics, offering significant advantages over traditional barrier membranes. In Guided Bone Regeneration (GBR) technology, titanium mesh provides strong support and protection for bone regeneration areas, promotes new bone formation and regeneration, and opens new possibilities for the repair of alveolar bone defects.
1. Mechanical Properties
Titanium mesh has notable mechanical advantages. Its tensile strength ranges from 100 to 140 kgf/mm², while its density is only 60% that of steel, meaning it provides powerful support while remaining lightweight. Additionally, titanium mesh has a low elastic modulus, low thermal conductivity, and is non-ferromagnetic, making it more adaptable and stable in use. In GBR technology, the stiffness, support, and retention capabilities of titanium mesh are critical, providing strong support and protection to prevent graft displacement and bone augmentation collapse.
2. Biological and Osteoconductive Properties
Titanium mesh also exhibits excellent biological and osteoconductive properties. First, it has excellent biocompatibility, making it well-tolerated by the body and unlikely to cause allergic or rejection reactions. In the human body, titanium mesh resists corrosion from secretions and is non-toxic, adapting well to sterilization methods, which is why it is widely used in medical devices and implants. Furthermore, while titanium mesh may release small amounts of metal particles, these do not significantly affect the growth rate of human cells, further proving its biological safety. Additionally, titanium mesh demonstrates superior mechanical properties and osteoconductive behavior, providing a stable space for bone regeneration and promoting the formation of new bone.
3. Excellent Physical and Chemical Properties
Titanium mesh inherits the corrosion resistance of titanium alloys, maintaining stability even in harsh environments, making it suitable for various corrosive conditions. Its surface can immediately form a uniform and dense oxide film that effectively resists corrosion from various agents. Moreover, titanium mesh has good breathability and flexibility, allowing it to be bent and shaped to fit the contours of different bone defects, providing greater flexibility in GBR procedures. Its macro-porosity plays a crucial role in maintaining blood supply and soft tissue attachment, which supports bone regeneration.
4. Good Biocompatibility and Malleability
Titanium mesh has excellent biocompatibility, with a smooth surface that is less prone to bacterial contamination, thereby reducing the risk of infection. This makes titanium mesh safer and more reliable in GBR applications. Additionally, titanium mesh offers a wide range of mechanical properties, and its thickness can be adjusted to meet different clinical needs. Surgeons can precisely shape and trim the mesh to fit the bone defect area, ensuring it provides effective support.
5. Differences from Other Membranes
Compared to traditional barrier membranes, titanium mesh offers significant advantages. Traditional membranes (such as absorbable collagen membranes or non-absorbable expanded polytetrafluoroethylene (ePTFE) membranes) provide cell-selective isolation, but their relatively soft nature makes it difficult to provide sufficient support and protection for the bone regeneration area. Titanium mesh, with its high strength, rigidity, and good biocompatibility, delivers better results in GBR applications. Additionally, the smooth surface of titanium mesh reduces the risk of bacterial contamination, lowering infection risks. However, the hardness of titanium mesh can also lead to potential issues such as mucosal irritation and exposure. Therefore, careful evaluation of the patient’s condition is necessary to choose the appropriate thickness and porosity of the titanium mesh.

Application of Titanium Mesh in Bone Augmentation
Titanium mesh GBR (Guided Bone Regeneration) technology was initially used primarily for the reconstruction of jawbone defects in maxillofacial surgery and later expanded to the field of alveolar bone defects. Since 1996, titanium mesh has been widely used in local alveolar ridge reconstruction due to its excellent biocompatibility and mechanical properties, meeting the requirements for initial implant stability and bone integration.
Specific Application of Titanium Mesh in Alveolar Ridge Reconstruction
According to the Terheyden classification, post-extraction alveolar ridge defects are divided into four types, with the application of titanium mesh varying based on the type of defect:
1/4 Defect: Early bone resorption, with the buccal bone loss being less than 50% of the anticipated implant length.
For this mild bone defect, traditional GBR technology can be used for bone augmentation.
2/4 and 3/4 Defects: Buccal bone resorption forms a blade-shaped alveolar ridge (2/4 type), or bone resorption after several years of tooth loss leads to a partial reduction in alveolar ridge height and width (3/4 type).
These moderate bone defects can be reconstructed using titanium mesh GBR. Depending on the degree of vertical resorption of the buccal or palatal bone walls, further subdivision into mild and severe cases is made. For mild resorption, GBR with titanium mesh can be performed alongside implantation. For severe resorption, delayed implantation, and bone augmentation with titanium mesh are more recommended.
4/4 Defect: After several years of tooth loss, bone resorption results in complete loss of alveolar ridge height and width.
For this severe bone defect, an onlay bone grafting method is recommended to address the need for reconstruction of the severely atrophied alveolar ridge.
Surgical Approaches of Titanium Mesh GBR Technology
Clinically, there are various surgical methods for titanium mesh bone augmentation, which can generally be categorized into: simultaneous implantation with titanium mesh bone augmentation, delayed implantation with titanium mesh bone augmentation, and titanium mesh combined with other bone augmentation techniques in GBR. When selecting the specific surgical method, it is important to thoroughly evaluate the patient’s overall health status and the alveolar bone defect in the surgical area to ensure a high success rate and patient comfort.
Application of Titanium Mesh in Simultaneous Implantation
In recent years, titanium mesh has been widely used in simultaneous implantation surgeries due to its excellent ability to maintain a stable bone formation space. Researchers have focused on exploring the application of titanium mesh in bone augmentation with concurrent implantation, aiming to reduce trauma and bone formation interference during secondary implant surgeries, thereby shortening the overall treatment process.
Titanium mesh has outstanding mechanical and biological properties, which means that the enhanced alveolar ridge typically does not experience significant resorption or deformation after the surgery. This characteristic provides strong support for simultaneous implantation. Both animal studies and clinical research have shown significant effects of titanium mesh in simultaneous implantation.
Numerous renowned dental experts have conducted extensive experiments, and the results indicate that titanium mesh is highly effective in simultaneous implantation. Under appropriate indications, titanium mesh bone augmentation followed by immediate implantation is a feasible and beneficial treatment strategy. This approach not only reduces surgical trauma and bone formation interference but also shortens the treatment process, improving patient comfort and satisfaction. Systematic reviews have also shown that the survival rate of implants placed simultaneously after titanium mesh bone augmentation is similar to that of implants placed immediately after bone grafting. No implant failures were observed in the statistical analysis.
Application of Titanium Mesh with Delayed Implantation
As a barrier membrane in Guided Bone Regeneration (GBR) technology, titanium mesh not only effectively promotes bone augmentation but also provides a solid foundation for the successful implantation of dental implants.
In practice, titanium mesh bone augmentation is often carried out in stages, separate from the implant placement. First, bone reconstruction is performed in the alveolar bone defect area, and once the bone tissue stabilizes, a second surgery is performed to precisely place the implant in the planned position. This staged treatment approach ensures a successful bone regeneration outcome and provides strong support for the success of the subsequent implantation surgery. Even if the initial bone augmentation surgery does not achieve the expected results, effective alveolar ridge reconstruction can still be accomplished in the follow-up implant surgery, ensuring the final implant success.
Numerous studies have confirmed the significant success of combining GBR technology with titanium mesh for bone augmentation in atrophic edentulous alveolar ridges. For example, a study by Poli et al. investigated the use of titanium mesh bone augmentation in edentulous patients with maxillary bone defects. After a 6-month recovery period, the patients achieved optimal bone regeneration, creating favorable conditions for implant success. Over an 88-month follow-up period, the amount of bone resorption in the newly formed bone was minimal, and no adverse effects on the implant function were observed.
Furthermore, research by Ciocca et al. further validated the effectiveness of GBR combined with titanium mesh for the reconstruction of extensive alveolar ridge defects. After 6 to 8 months of recovery, patients achieved an average vertical bone gain of (3.89±1.46) mm, fully meeting the implant requirements of the reconstructed site. However, it is important to note that large-scale bone augmentation surgeries are often associated with higher exposure rates and failure rates. In Ciocca’s report, the exposure rate of titanium mesh was as high as 66%, but fortunately, these exposure cases did not significantly impact the bone augmentation outcome, highlighting the unique advantages of titanium mesh in dental implantology.
In conclusion, the application of titanium mesh with delayed implantation has brought revolutionary changes to the field of dental implants. It not only increases the success rate of bone augmentation but also provides a stronger foundation for the successful placement of dental implants.
Application of Titanium Mesh Combined with Other Bone Augmentation Methods
When dealing with extensive alveolar bone defects or complex bone wall deformities, the application of titanium mesh combined with other bone augmentation methods has shown significant advantages in the field of dental implants.
Using high-density bone grafts or Guided Bone Regeneration (GBR) technology alone may face risks such as compensatory bone resorption or barrier membrane exposure. The use of titanium mesh can also reduce the amount of autogenous bone required for high-density bone grafting, providing greater convenience and comfort for the patient.
Titanium mesh bone augmentation can even play a crucial role in the reconstruction of alveolar bone defects after failure of high-density autogenous bone grafts. Studies have shown that using titanium mesh to cover hydroxyapatite particles for reconstructing severely resorbed alveolar bone after the failure of high-density grafting still resulted in an average bone gain of (4.2±0.5) mm. After a 3-year follow-up, the bone tissue around the implant remained stable, further confirming the effectiveness and stability of titanium mesh bone augmentation. Additionally, GBR with titanium mesh can also be combined with tenting bone enhancement techniques to further improve the bone augmentation outcome.
The application of titanium mesh combined with other bone augmentation methods in dental implantology has significant advantages. It not only reduces the risk of surgical failure and bone resorption but also decreases the need for autogenous bone, providing better treatment outcomes and increased comfort for patients.
Titanium mesh bone augmentation surgery
Bone Grafting Materials
Bone grafting materials play a crucial role in dental implant and bone reconstruction surgeries. Granular bone grafts are considered ideal filling materials because they can fill various shapes of bone defects and prevent the formation of gaps under the titanium mesh. Compared to block bone grafts, granular bone grafts have better osteogenic and osteoinductive properties. Autograft materials collected using a Bone Scraper, combined with the use of titanium mesh, have shown significant results in bone reconstruction and implant survival.
However, the use of autogenous bone is limited by the bone quantity at the donor site, surgical costs, and the patient’s condition. To overcome these limitations, researchers have explored mixing autogenous bone with xenografts (such as inorganic bovine bone material, DBBM) to reduce the demand for autogenous bone. DBBM, one of the most commonly used xenograft materials, has high biocompatibility and low resorption rates. It can integrate with new bone, maintain the graft volume, and increase bone density after bone formation.
Many studies have investigated the use of xenografts or mixtures of autogenous and xenograft bone for bone augmentation. In clinical studies, the ratio of autogenous to xenograft bone is typically 50/50 or 30/70. Although histological studies suggest that increasing the proportion of autogenous bone in the graft mixture leads to a higher proportion of new bone, no statistically significant difference has been found to prove the critical role of autogenous bone in the graft mixture. This indicates that in titanium mesh bone reconstruction, similar and predictable results can be achieved with either autogenous bone or different xenograft materials.
In recent years, bone induction factors based on recombinant human bone morphogenetic protein (rhBMP), due to their ability to promote bone reconstruction and formation, have become alternatives to traditional bone grafting. Among these, rhBMP-2 has been shown to have a high osteoinductive potential, playing an important role in the early stages of mesenchymal stem cell proliferation and differentiation into osteoblasts. However, because rhBMP-2 is mainly stored in liquid form, it requires the use of absorbable collagen sponges (ACS) as a carrier. However, collagen carriers lack structural stability, which often requires the use of titanium mesh in horizontal or vertical bone augmentation with rhBMP-2/ACS to prevent collapse of the osteogenic space under pressure.
Although clinical studies indicate that there is no significant difference in horizontal bone augmentation using rhBMP-2 compared to autogenous bone grafting, the osteointegration process induced by rhBMP-2 takes longer. Additionally, the release process of rhBMP-2/ACS is uncontrollable, which may lead to adverse reactions such as postoperative edema. Therefore, the potential risks and benefits of using rhBMP-2 for bone augmentation need to be carefully weighed.
Whether using autogenous bone, xenograft materials, or rhBMP-based bone induction factors, they can all be combined with titanium mesh to achieve good bone augmentation outcomes. However, when selecting specific bone grafting materials and protocols, a comprehensive consideration of the patient’s individual condition, surgical requirements, and expected outcomes is necessary.
Covering Materials
Titanium mesh plays a crucial role in bone augmentation surgery, providing a scaffold for tissue growth due to its porous structure. However, it can also lead to excessive soft tissue formation underneath the mesh. To close the pores of the titanium mesh and optimize the healing process, researchers have explored the use of various covering materials.
Absorbable collagen membranes are commonly used due to their excellent biocompatibility and ability to promote mucosal healing. However, a retrospective cohort study found that there was no statistically significant difference in titanium mesh exposure rates with or without collagen membrane coverage, and dense fibrous tissue was still observed beneath the titanium mesh. This suggests that absorbable collagen membranes have limited efficacy in reducing soft tissue formation under the titanium mesh.
In contrast, the use of concentrated growth factors (CGF) has shown more promising results. CGF is placed between the titanium mesh and gingival soft tissue to promote angiogenesis and the differentiation of fibroblasts. This accelerates soft tissue healing, protects the titanium mesh and bone graft materials beneath the gums, and reduces inflammatory responses. A clinical cohort study indicated that patients receiving CGF treatment showed better healing during titanium mesh bone augmentation and had a lower exposure rate of the titanium mesh.
Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are also used in titanium mesh bone augmentation surgeries. PRP has a higher platelet concentration but relatively low natural fibrinogen content. A randomized controlled trial demonstrated that the titanium mesh group using PRP showed better outcomes in terms of complications and bone formation, with no observed titanium mesh exposure. PRF, as a second-generation platelet concentrate, is easier to prepare and apply compared to PRP. A retrospective study showed that covering the titanium mesh with solid advanced PRF (A-PRF) significantly reduced the exposure rate of the titanium mesh. This may be related to the initial clot stability mediated by fibrin in PRF, as well as its ability to stimulate osteoblasts, periodontal ligament cells, and epithelial cells, promoting bone defect repair, wound healing, and microvascularization.
The application of different covering materials in titanium mesh bone augmentation surgeries yields varying results. Biological materials such as CGF, PRP, and PRF have gained attention due to their ability to promote soft tissue healing and reduce complications. These materials offer additional options and possibilities for enhancing the outcomes of titanium mesh bone augmentation surgeries.
Fixation of titanium mesh
In vitro studies have shown that blood, upon contact with the surface of titanium mesh, releases migration factors that play a crucial role in the early stages of cell recruitment. The interaction between blood and titanium mesh helps regulate the early osteogenic microenvironment during bone healing and remodeling. In guided bone regeneration (GBR) procedures involving titanium mesh, it is essential to ensure firm fixation of the mesh to prevent micro-movement, which could impact thrombus formation.
Depending on the patient’s specific bone augmentation needs and whether simultaneous implant placement is planned, various methods can be used to secure the titanium mesh.
- Single Tooth Defect with Simultaneous Implant Placement In cases of single-tooth defects with planned simultaneous implantation, absorbable sutures can be used to fix the titanium mesh. This method avoids the potential impact of titanium screws on the implant and reduces the need for subsequent surgeries to remove the mesh and screws, thus minimizing patient trauma.
- Delayed Implantation for Single Tooth Defect In delayed implantation for single-tooth defects, where there are no restrictions from the implant during the two-stage procedure, titanium screws can be used to secure the titanium mesh. The screws and mesh can be easily removed during the subsequent implantation surgery.
- Multiple Tooth Defects For patients with large bone defects involving multiple teeth, the titanium mesh may experience movement due to the size of the defect. To ensure stability of the blood clot and wound healing, titanium screws are typically used to firmly secure the mesh between multiple implants while ensuring that the implants themselves are not affected.
- Pre-fabricated Titanium Mesh in Digital Procedures For titanium mesh that has been pre-fabricated through digital processes, it can be directly fixed to the implant using appropriate implant systems with corresponding accessories such as height adjusters, caps, or healing abutments. These accessories can be used for both submerged and non-submerged implant placements, depending on the case.
- Additional Fixation Methods To more effectively fix the titanium mesh, specialized titanium mesh fixation Kit can be used, or a combined support srews model may be applied. The choice of fixation method should be based on the patient’s specific situation and surgical requirements.
The fixation of titanium mesh plays a critical role in the success of bone augmentation surgeries, and various methods can be employed to ensure the stability and healing of the bone and surrounding tissues. The fixation method chosen should be tailored to the patient’s condition and the specific needs of the surgery.
Complications, Management, and Prevention of Dental Titanium Mesh
I. Complications of Dental Titanium Mesh
- Infection: After titanium mesh implantation, if oral hygiene is inadequate or the surgical area is contaminated, infection may occur. Infection may present with local redness, swelling, pain, fever, and in severe cases, it can compromise the stability of the titanium mesh and the healing process.
- Mesh Exposure: Titanium mesh may become exposed to the oral environment due to improper fixation or poor soft tissue healing. Exposed mesh can increase the risk of infection and affect the patient’s oral function and aesthetics.
- Soft Tissue Proliferation: Due to irritation from the titanium mesh or poor oral hygiene, soft tissue proliferation may occur, leading to granulomas or polyps. Soft tissue proliferation may affect the patient’s chewing function and oral comfort.
- Nerve Injury: Improper handling during titanium mesh implantation can damage nearby nerves, leading to sensory abnormalities such as numbness in the lower lip.
- Mesh Displacement or Loosening: If not fixed securely or if the patient misuses it, the titanium mesh may shift or loosen. Displacement or loosening of the mesh can affect its functionality and aesthetic outcome.
II. Management of Dental Titanium Mesh
- Postoperative Monitoring: Regular oral and radiographic examinations should be conducted to monitor the stability and healing of the titanium mesh. Any complications such as infection or mesh exposure should be promptly identified and treated.
- Oral Hygiene Maintenance: Patients should be guided on proper brushing techniques and the use of dental floss to maintain oral hygiene. Avoid using overly harsh oral hygiene products that could damage the oral mucosa.
- Dietary Guidance: Advise patients to avoid hard, sticky, or irritating foods that could damage the titanium mesh or interfere with healing.
- Regular Follow-up Visits: Schedule regular follow-up appointments to identify and manage any potential complications early.
III. Prevention of Dental Titanium Mesh Complications
- Preoperative Assessment: Prior to titanium mesh implantation, a comprehensive oral and general health assessment should be conducted. Assess the patient’s oral hygiene status, jawbone defects, overall health, and determine if they are suitable candidates for titanium mesh implantation.
- Intraoperative Care: Adhere to strict aseptic techniques during surgery to prevent contamination of the surgical area. Operate carefully to avoid damaging nearby nerves and blood vessels. Choose the appropriate size and shape of titanium mesh to ensure it fits perfectly with the jawbone defect.
- Postoperative Care: Provide patients with detailed postoperative care instructions, including oral hygiene maintenance and dietary guidance. Inform patients about possible discomforts and complications, and teach them how to identify and manage them.
- Use of Biological Materials: Consider using biological materials such as concentrated growth factors (CGF) or platelet-rich plasma (PRP) during titanium mesh implantation to assist in healing. These biological materials promote soft tissue healing and reduce the risk of mesh exposure and infection.
Future Trends of Titanium Mesh in Bone Augmentation
Titanium mesh will continue to develop in the direction of technological innovation, personalized customization, enhanced biocompatibility and functionality, and an expanded clinical application range. With continuous technological advancements and deeper clinical applications, titanium mesh will play an increasingly important role in the field of bone augmentation.
I. Technological Innovation and Personalized Customization
- 3D Printing Technology:
3D printing technology enables the customization of titanium mesh based on the specific needs of patients. This personalized mesh can more accurately fit the bone defect area, improving the precision and success rate of surgeries.
3D printing can also create complex geometries and microstructures, optimizing the mechanical and biological properties of titanium mesh. - Digital Design and Simulation:
Digital design software allows surgeons to plan and simulate surgeries with high precision before the operation, reducing uncertainties and risks during the procedure.
Digital technologies also help surgeons better assess the compatibility of titanium mesh and the expected surgical outcome, enhancing the reliability and safety of the surgery.
II. Enhancements in Biocompatibility and Functionality
- Surface Modification Technology:
Modifying the surface of titanium mesh, such as by spraying bioactive ceramic coatings or applying micro/nanostructuring, can improve its biocompatibility and bone integration capabilities.
These surface modifications promote the adhesion, proliferation, and differentiation of osteoblasts, accelerating new bone formation and the healing process. - Functional Composite Materials:
Combining titanium mesh with other functional materials, such as growth factors or drug delivery carriers, can endow the mesh with additional biological functions.
These composite materials can promote bone tissue regeneration and repair while reducing inflammation and infection risks.
III. Expansion of Clinical Application Range
- Repair of Complex Bone Defects:
With ongoing advancements in technology, the application of titanium mesh in the repair of complex bone defects will become more widespread.
Whether it’s horizontal or vertical bone defects, titanium mesh can provide effective support and act as a barrier, promoting the formation and healing of new bone. - Interdisciplinary Applications:
The application of titanium mesh in bone augmentation will not be limited to oral medicine; it can also be expanded into other medical fields.
For instance, in orthopedic surgery, titanium mesh can be used to repair bone defects following fractures or joint replacement. In plastic surgery, titanium mesh can be used to reconstruct facial bones or repair soft tissue defects.
Conclusion
Guided Bone Regeneration (GBR) technology uses titanium mesh as a barrier membrane, effectively preventing non-osteogenic cells from invading the bone defect area and providing a regeneration channel for osteogenic cells. Due to its high strength, excellent biocompatibility, and osteoconductivity, titanium mesh performs exceptionally well in the repair of severe alveolar bone defects. Since 1996, titanium mesh GBR technology has been widely applied in local alveolar ridge reconstruction and is suitable for various degrees of post-extraction defects. When combined with other bone augmentation methods and biomaterials, titanium mesh reduces the risk of surgical failure and bone resorption. With technological advancements, the application of titanium mesh will continue to expand, offering more possibilities for the repair of complex bone defects and interdisciplinary applications.
FAQ
Q1: What are the disadvantages of titanium meshes?
A: The main disadvantages of titanium meshes include: their hardness, which may cause mucosal irritation or exposure; in some cases, the titanium mesh may require a secondary surgery for removal; additionally, the cost of titanium meshes is relatively high, which may increase the economic burden on patients.
Q2: Will bone grow on the titanium mesh?
A: Yes, bone can grow on titanium meshes. Titanium meshes have good osteoconductive properties, meaning they promote the attachment, proliferation, and differentiation of bone cells on their surface, leading to the formation of new bone tissue.
Q3: Why is bone grafted onto titanium mesh?
A: Bone is grafted onto titanium meshes to provide additional bone volume in bone defect areas, promoting bone regeneration and repair. Titanium meshes act as a scaffold, supporting and guiding the growth of new bone, thereby restoring the structure and function of the bone.
Q4: Will titanium fuse with bone?
A: Yes, titanium can fuse with bone. Titanium forms osseointegration with bone tissue, where bone cells attach and proliferate on the surface of titanium, allowing the titanium mesh to securely fix itself in the bone defect area.
Q5: Will titanium mesh dissolve?
A: Titanium mesh will not dissolve. Titanium is a metal with excellent corrosion resistance and stability, allowing it to remain in the human body long-term without undergoing chemical changes or dissolving.
Q6: What are the disadvantages of titanium in dentistry?
A: Disadvantages of titanium in dentistry may include: its hardness can cause discomfort during use; in some cases, titanium may cause allergic reactions; additionally, the cost of titanium may be relatively high, increasing the treatment costs for patients.
Q7: Should I remove the titanium mesh?
A: Whether the titanium mesh needs to be removed depends on the patient’s specific situation. If the titanium mesh has not caused any discomfort or complications and has successfully promoted bone regeneration and repair, removal is typically not required. However, if the mesh causes mucosal irritation, exposure, or other issues, removal or other treatment options may need to be considered. The decision should be made after thorough discussion and evaluation with the doctor.