Home / 会社概要
DentalMaster is a subsidiary of Double Medical, which was listed on the Shenzhen A-share market in 2017 (stock code: 002901). The company has obtained certificates such as FDA, CE, ISO13485, and GMP, ranking the first in China in the fields of trauma and neurosurgery.
Established in 2010, DentalMaster focuses on developing high-quality dental implants. Through collaboration with Xiamen University, it integrates industry, academia, and research, combining advanced technical R&D teams and top manufacturing processes to offer implant systems, surgical cutting products, and personalized digital restoration solutions.


Since 2010, DentalMaster has been providing comprehensive solutions to the global dental industry. DentalMaster implants are made from Cold Working Titanium Grade 4, a material known for its high biocompatibility and mechanical strength, effectively avoiding the toxicity associated with some metals. The raw materials used by DentalMaster come from the same renowned supplier as Nobel Biocare—US Carpenter Technology Ltd.
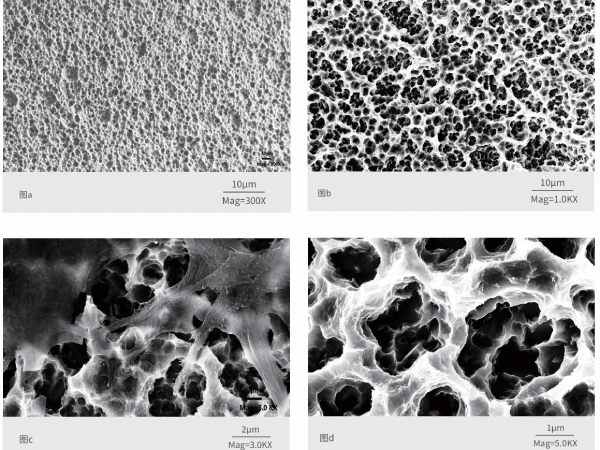
Our unique SLA surface treatment creates a multi-layered microstructure (20-30μm and 1-10μm) and is visible under electron microscopes at 500x and 3000x magnification. With a surface roughness under 0.2 microns, our implants ensure precision. They have passed anti-fatigue tests, simulating 10 years of use and over 5 million chewing cycles.
DentalMaster’s unique design and revolutionary clinical techniques have stood the test of time. We invite clinicians worldwide to experience the benefits of DentalMaster implants.
DentalMaster was established with a focus on delivering high-quality, reliable dental implant solutions. We commit to precision manufacturing and use premium materials. Cutting-edge technology ensures implants that promote faster healing and long-lasting results. DentalMaster’s mission is to offer dentists around the world the tools they need to provide the best care for their patients, ensuring confident smiles and successful outcomes.
DentalMaster has obtained China CFDA, CE, and ISO 13485 system certificates, and patented SLA surface treatment technology
The company is listed on the stock exchange with the stock code: 002901.
DentalMaster was recognized as a national high-tech enterprise and gained market access in dozens of countries, including Europe, the U.S., and Southeast Asia, receiving relevant certificates.
DentalMaster is rapidly expanding and can be seen at dental professional exhibitions in Europe, the Middle East, Malaysia, Thailand, the Philippines, and other countries.
Our implants are made from high-quality titanium, produced with advanced manufacturing processes, and come with a lifetime warranty.

For decades, DoubleMedical has focused on orthopedic rehabilitation, accumulating extensive expertise and investing in research and advanced technology, driving continuous innovation and progress in the field of dental implants.
SLA big particle sandblasting and acid etching create a complex surface, increasing bone integration and promoting faster osteogenesis. The smooth, multi-micron active surface with smaller contact angles helps induce new bone formation.
Cold Working Titanium Grade 4 has excellent strength and corrosion resistance, allowing it to maintain long-term stability in the harsh oral environment. The cold working process further enhances the material’s mechanical properties, giving it higher tensile strength and better wear resistance. Additionally, Grade 4 titanium offers exceptional biocompatibility, integrating well with human tissue, which contributes to the rapid healing and long-term success rate of implants, making it an ideal choice for high-performance implant materials.
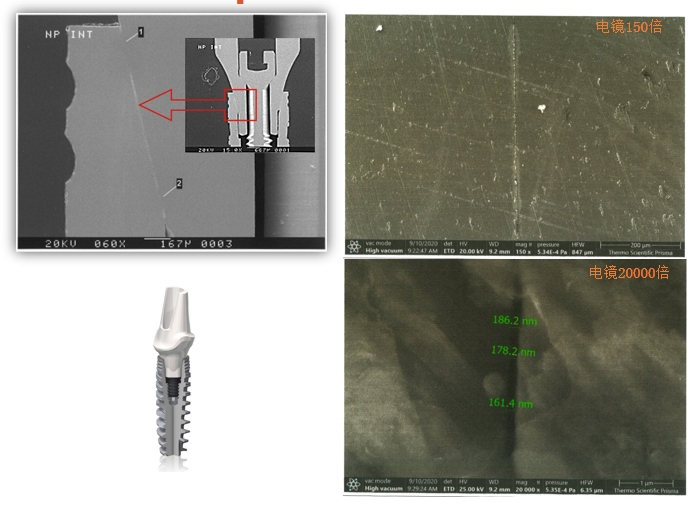
Under the electron microscopy 150x and 20000x, the gap between DentalMaster and abutment is smaller than 0.2 micron and lower than similar products in the industry, which shows the higher processing accuracy, better anti-fatigue and anti-deformation, and longer the duration period.
Our dental products meet strict quality regulatory standards, and we are committed to continuous learning and improvement to ensure that both you and your dental patients can receive the highest quality and most professional products and services available on the market.

CE certification (click to view)

ISO13485 certification (click to view)

DENTALMASTER SLA Dental Implant System_Product Approval_Indonesia Exp. 12102025 (click to view)

Product license 67-2-2-2-0004127_DentalMaster Dental Implant System (click to view)
デンタルマスター は、インプラント歯科を専門とする会社である。Nobel Biocare, Short Bicon, Straumann, Zimmer, Megagen, Dentium, Hiossenなどのブランドは各社の登録商標です。DentalMasterはこれらの企業と商業的な提携関係はありません。これらのブランドは、DentalMasterが提供する互換性のあるインプラント、インスツルメント、アバットメント、コンポーネントを正確に特定する目的でのみ言及されています。
著作権©2024デンタルマスターCE / ISO13485。すべての権利予約.